3CLpro
A Small Molecule Inhibitor of 3CLpro as a Potential Treatment of COVID-19 (Phase I Completed)
Assays Completed
Wholly-owned and Available for Licensing
In vivo efficacy studies –
SARS-CoV-2 Omicron BA2.3 variant
SARS-CoV-2 Omicron BA2.3 variant
In vivo efficacy studies –
SARS-CoV-2 WT variant
SARS-CoV-2 WT variant
In vivo efficacy studies – MERS-CoV
In vivo PK studies
In vitro ADMET studies
Developability/CMC
In vitro cell-based
Toxicology studies
Enzymatic
Target Rationale
The coronavirus protease 3CLpro is conserved in structure and function in all known coronaviruses and serves as the main protease for proteolytic processing of the replicase polyproteins, as the name "main protease" refers to the critical role. In addition, the active-center amino acids of 3CLpro have low homology with human.
Thus, inhibition of 3CLpro has been proven to be an effective treatment to block SARS-CoV-2 replication and further to treat multiple strains of COVID-19 disease and other coronaviruses.
Thus, inhibition of 3CLpro has been proven to be an effective treatment to block SARS-CoV-2 replication and further to treat multiple strains of COVID-19 disease and other coronaviruses.
Insilico Medicine 3CLpro Inhibitor Summary – Phase I
Pan-coronavirus activity
- Strong inhibition against all human coronavirus 3CLpro
- Broad antivirus activity against multiple strains & variants
- Possibility to overcome Nirmatrelvir clinical resistance
Novel binding mode Lower efficacious dose
- Kinact/Ki suggests high efficiency of covalent bond formation
- Enzymatic dilution assay confirms the unique irreversible binding nature
- Enables different PK/PD correlation
Promising druggability Oral single agent
- Superior passive permeability in Caco-2 assay compared to Nirmatrelvir
- Lower efficacious dose in mouse models
- No CYP/transporter inhibition concern
High synthetic feasibility
- 2 chemical step synthesis from starting materials with simple structure
- Practical API production
Indication
By February 2023, more than 700 Million confirmed cases of COVID-19, including 6 Million deaths globally were reported by World Health Organization (WHO). The real number of deaths was likely underestimated given the wide spread of Omicron (BA.5, BA. 2.75 and XBB 1.5).
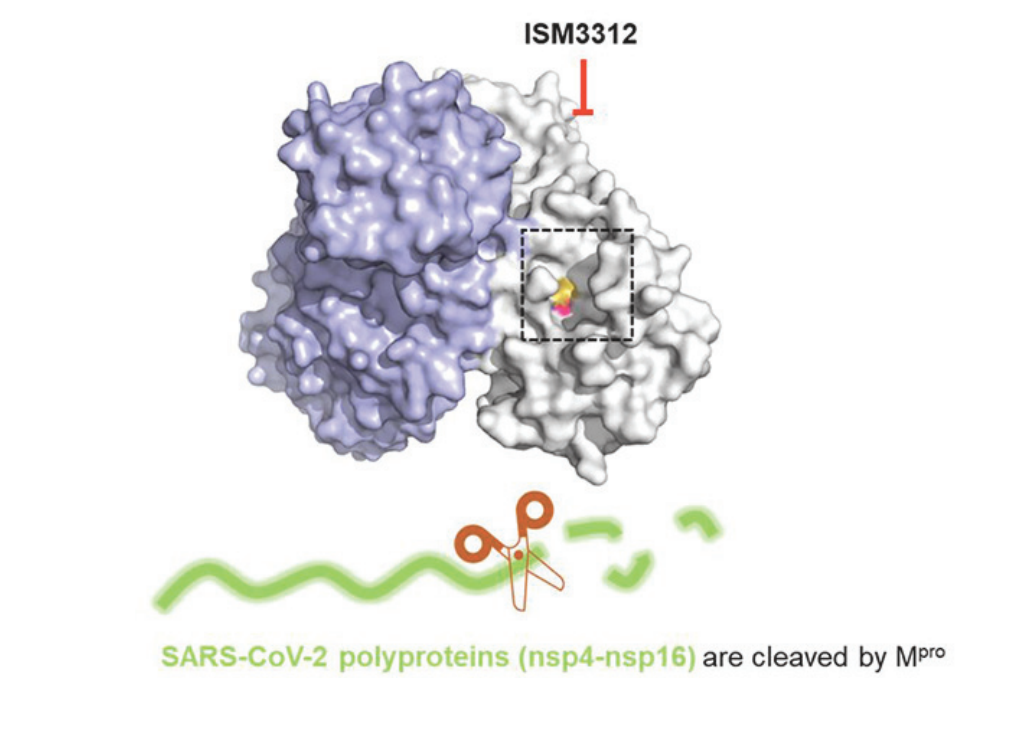
3CLpro is highly conserved in the genus coronavirus including MERS-CoV, SARS-CoV and other respiratory coronaviruses and its inhibition blocks the release of nsp4 to nsp16, especially nsp12 (also RdRp) that are essential for SARS-CoV-2 replication. This makes 3CLpro an attractive target for treatment of COVID-19. Moreover, given the 3CLpro enzyme's role in viral replication, its potential for mechanistic safety, and expected lack of spike protein resistance variant challenges, its inhibition by a small molecule inhibitor seems attractive.
3CLpro is highly conserved in the genus coronavirus including MERS-CoV, SARS-CoV and other respiratory coronaviruses and its inhibition blocks the release of nsp4 to nsp16, especially nsp12 (also RdRp) that are essential for SARS-CoV-2 replication. This makes 3CLpro an attractive target for treatment of COVID-19. Moreover, given the 3CLpro enzyme's role in viral replication, its potential for mechanistic safety, and expected lack of spike protein resistance variant challenges, its inhibition by a small molecule inhibitor seems attractive.
Project Status – Phase I Completed
An orally available, irreversible covalent inhibitor of 3CLpro, also called 3CL protease or main protease ("Mpro"), which is a conserved cysteine protease and an essential enzyme for the replication of severe acute respiratory syndrome coronavirus 2 ("SARS-CoV-2"), the causative agent of COVID-19.
The preliminary results demonstrated broad antiviral activity against other coronavirus and potential clinical drug resistances. We received the IND approval in February 2023 and initiated a Phase I clinical trial in China in March 2023.
The preliminary results demonstrated broad antiviral activity against other coronavirus and potential clinical drug resistances. We received the IND approval in February 2023 and initiated a Phase I clinical trial in China in March 2023.

Insilico Medicine's (ISM) compound showed promising anti-virus effect and improved lung histopathology effect in SARS-COV-2 hACE2 transgenic mice model
